
IND ETO C1445 Industrial Ethylene Oxide Gas Sterilization Devices Brochure EN - Pdf 692 Kb.
IND ETO C1445 Industrial Ethylene Oxide Gas Sterilization Devices Catalogue EN - Pdf 9166 Kb.
| ISO 9001 | ISO 13485 | CE 1984 |
IND ETO C1445 Industrial Ethylene Oxide Sterilizer Data Sheet EN - Pdf 529 Kb.

Industrial Type EO Suture Sterilizer ETO-C 1445 is the best solution partner for suture and medical equipment manufacturers. Most products that are sensitive to heat and humidity can be sterilized with Ethylene Oxide gas sterilization. ETO-C 1445 will be the most important building block of your production line with different M3 options.
Teknomar Ltd follows a customer-oriented approach to be the best solution partner.
Sterilization should be considered the first and most important stage of production.

Teknomar Ltd has undertaken professional projects with its experienced engineers and technicians. Standard and Special Production devices are manufactured from the highest quality stainless steel.
Teknomar Ltd has strong experience in the sterilization of disposable medical products, absorbable and non-absorbable sutures, catheters, laparoscopic surgical instruments, implants, sensitive flexible medical products, endosppositories, rigid and semi-rigid lumen-shaped instruments.



Different Gas Mixtures Can Be Selected According to the Material Validation. Some of them;
| Industrial Type EO Sterilization Device C-1445 | Ethylne Oxide Sterilization Device Chamber Inner Dimensions |
Weight (~kg) |
Power (kW) | ||||
| Chamber Volume (~m3) | Euro Pallet | Door | Width (cm) | Depth (cm) | Height (cm) | ||
| 0,5 Suture | - | Single | 82,5 | 74 | 82,5 | 960 | 10 kW |
| 1 | - | Single | 90 | 110 | 100 | 1450 | 21 kW |
| 1,6 Suture | - | Single/Double | 90 | 158 | 113 | 2000 | 25 kW |
| 2,1 Suture | - | Single/Double | 90 | 160 | 140 | 2500 | 30 kW |
* The above dimensions show the standard production. The custom sized manufacturing is available, Please contact to your representative for more information.
* Pallet size: EURO 80 X 120 cm
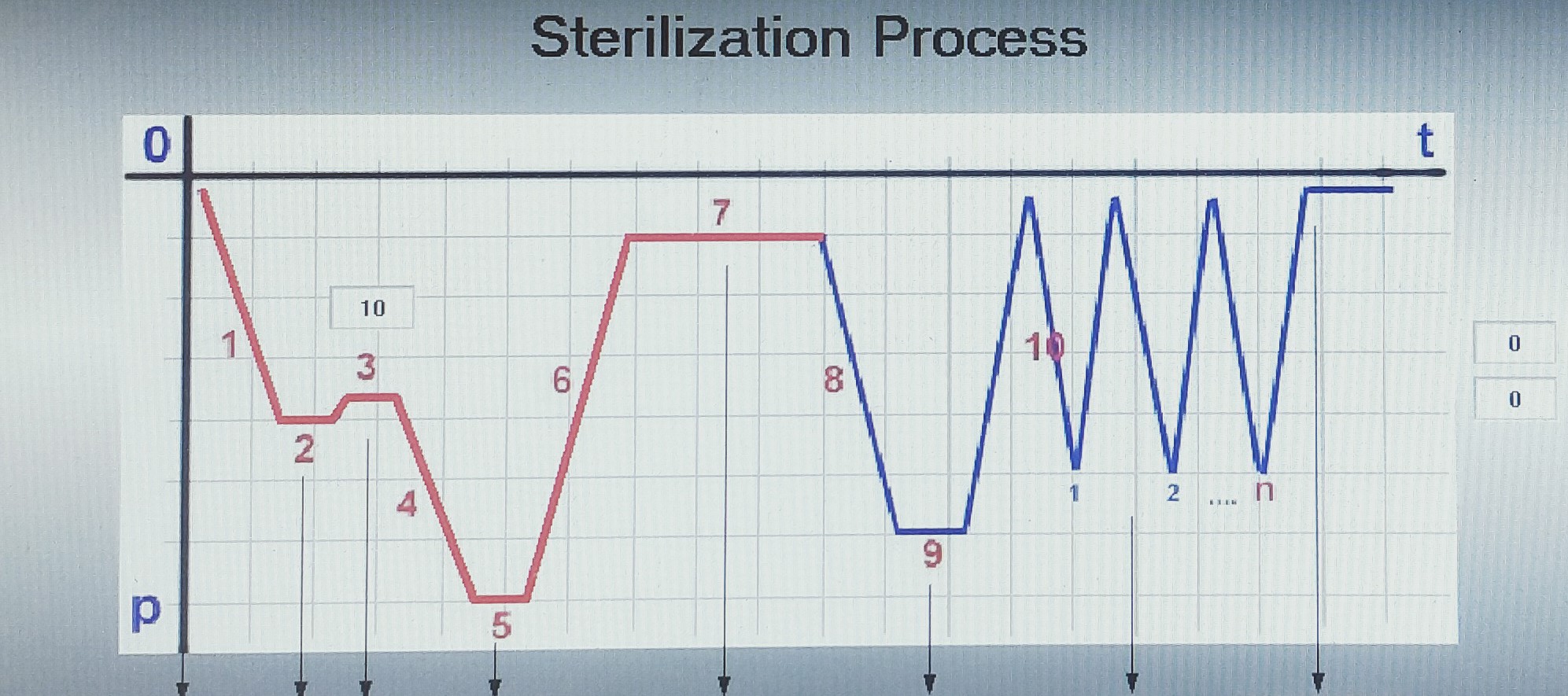
ETO C1445 Suture Sterilization device has a compact one-piece built-in design for the user to use for multifunctional purposes. The device allows the user to program different parameters for multiple suture sterilization.
In today's low-tech devices, users have to use separate devices for preconditioning, dehumidification and drying of products (Bagged and Ampoule Breaking, Old Technology under Vacuum). This increases the workload of employees, sterilization time and sterilization cost.
ETO C1445 offers a new technique in suture sterilization where preconditioning, sterilization, ventilation, dehumidification and drying processes are performed by only one device.


Once the ETO C1445 suture sterilization processes are completed, the sterile packaged products can be used immediately without the need for additional ventilation, as there is no EO gas residue on the products.
ETO C1445 suture sterilization reduces all possible costs to provide you with optimized economies of scale. Teknomar Ltd. offers verification and calibration services for different materials. The average sterilization time is 6-8 hours excluding drying processes, although this time can be lower or higher depending on the material to be sterilized. FAT (Factory Acceptance Test). Teknomar Ltd. assigns its best programmers and technicians to set the device to the most suitable sterilization level, considering the sterilization cycle costs and sterilization accuracy.






Siemens, Festo, ABB, AdvanTech, Oksitem, Yeni Kromsan, Schneider, Phoenix, Reliance
ETO C1445 maintenance is simple and easy and does not require professionalism. Daily and routine maintenance can be easily done by trained users.
Teknomar Ltd provides design, manufacturing, assembly, training, service, validation and related services to its customers.
Information Form
Top of Page
Warnings: